Baxter’s New Treatment for Alzheimer’s Delays, Partly Reverses Disease!
by Aaron Saenz April 15th, 2010 | Comments (2)
There are few diseases that are as terrifying as Alzheimer’s. Losing your memory, your comprehension, and knowing all the while that your life is slowly fading from your mind. It’s a very scary reality for the 30 million people on the planet who have it. To date there are no real ways to cure the illness. Luckily, help may be on the way. Baxter International (NYSE:BAX) released theresults from its phase II clinical trial on April 13th, demonstrating that their Gammagard product could help stop the loss of brain cells in Alzheimer’s patients. That’s phenomenal! This is a treatment that could stop, maybe even reverse, some of the progression of Alzheimer’s disease, and it’s already been tried in humans. It will likely be several years until Gammagard is ready to hit the market (there are still phase III trials to pass) but we have gotten closer to an effective means of slowing or stopping Alzheimer’s in its tracks, and that’s incredible news.
The recently completed Baxter study, which was performed in cooperation with the Weiss Cornell Medical Center, looked at 14 patients over 18 months using MRI scans to record the rate of brain cell loss. According to their press release, those patients on Gammagard experienced less loss of brain cells (measured by swelling in areas of the brain) than those patients on placebo. Cognition and function was also better for Gammagard patients than those on placebo. These results are pretty amazing. Losing fewer brain cells is high on my list of positive effects for any drug and preventing Alzheimer’s related memory and cognition loss is also damned important. According to Bloomberg, there were even some patients that showed noticeable reversal in Alzheimer’s symptoms. One patient, a pianist, was formerly only able to remember and play about four songs. After the 18 month trials, family members reported that the patient had started to learn new songs. That’s news that brings hope to Alzheimer’s patients everywhere and would certainly make selling Gammagard very easy.
The market for Alzheimer’s drugs is already in the billions. Aricept, from Pfizer, had sales in excess of $3 billion last year, and Forest Labs sold $950 million of their product, Numenda. The market is driven by the 30 million Alzheimer’s patients in the world, 5 million or so in the US.According to the CDC there may be around 13 million patients in the US by 2050. Medicare spent $91 billion in Alzheimer’s related expenses in 2005 alone. The world population is continuing to get older, and Alzheimer’s is an age-related disease. Overcoming it will be a major hurdle in our pursuit of longevity.
Gammagard is a immunotherapy product (intravenous immunoglobulin or IVIG) developed from thousands of human plasma donations. It’s been FDA approved (for immunotherapy) and on the market since the 90s in various forms. Many believe that Alzheimer’s may be caused by the buildup of amyloid plaque and related inflammation on the brain, and Gammagard contains amyloid fighting antibodies. In the phase II trials nurses traveled to the residences of patients and gave them Gammagard intravenously with regularly scheduled check ups and brain scans to measure effect. Those on placebo were switched to the drug after six months.
According to Baxter, the recently completed phase II trial for Gammagard is the first that tracked the trifecta for Alzheimer’s treatment: neuro-imaging, cognition, and brain function. Baxter plans on continuing with two phase III trials, the first of which is already started, and the second will begin by late 2010. This second phase III trial will continue to use MRI scans. The first phase III trial will focus on cognition and function and is part of the Gammaglobulin Alzheimer’s Partnership (GAP) study. 360 patients at 35 sites across the country (12 more sites pending in the US and Canada) will be studied for 18 months to see how Gammagard affects the progression of Alzheimer’s Disease. GAP is still actively recruiting patients, and those interested can check their website for eligibility. Because these studies will take at least 18 months to run, the earliest we will see results is likely to be 2012 for the GAP study and 2013 for the other phase III trials. In other words, there are years before we could see Gammagard approved by the FDA.
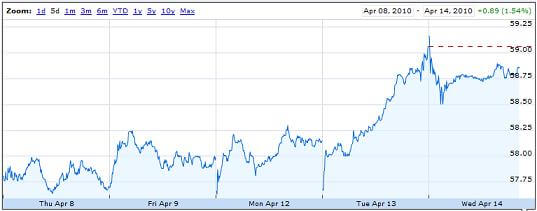
That being said, it’s important to note that Baxter is not the only company developing an IVIG drug for Alzheimer’s. Octapharma has its own phase II trial for octagam, a product much like Gammagard in basic composition. According to ClinicalTrials.gov, Pfizer and GSK also have intravenous treatments for Alzheimer’s Disease in the pipeline, PF-04360365 and GSK933776 respectively, both of which are anti-amyloid, though not necessarily immunoglobulin. Despite the competition, Gammagard is arguably the closest of these products to obtaining FDA approval and introduction into the market though Baxter’s stock rise after its announcement on April 13th was surprisingly small.
If I was an Alzheimer’s patient, or a family member of one, I’d probably be very interested in getting my hands on this product now. And you can: Gammagard and other IVIGs are already on the market for other uses. Undoubtedly there will be some doctors and patients out there who decide to use them for off-label treatments of Alzheimer’s. I’m not a doctor so I can’t comment on whether or not that’s a good idea. I will say, however, that as remarkable as these early findings may be, the sample sets were very small: just 14 people. The phase III trials will have many more patients and large studies can often show complications or contradictions to smaller studies, so we shouldn’t consider this work proven as of yet. Still, even these early results are very exciting.
Obviously any positive results for a new Alzheimer’s drug is good news. It’s hard to know, however, if Gammagard (or other IVIG products) will be a catch-all therapy, or just one drug in a cocktail used to treat the illness. The latter is much more likely. While I applaud trials like this one, they are only part of a much larger suite of research that has to be performed in order for us to understand and defeat Alzheimer’s. We’ve seen how genetic insights are helping us determine the risks for the disease, and genomics may allow us to identify individuals at risk much earlier in their lives. I also have hope that with the billions in sales (and millions in research), we’ll eventually determine the exact cause and develop an outright cure for the condition. Until then, I wish the best to Baxter and Cornell in their clinical trials and urge them to develop this new weapon against Alzheimer’s as soon as possible.
No comments:
Post a Comment